Frequently Asked Questions About Clinical Trials
WHY SHOULD YOU PARTICIPATE?
When current treatment is not working and alternative medications are not available, a patient may have access to medications otherwise unavailable. Through participation you will take a more active role in the control of your disease state. By participating, you will help others by contributing to the advancement of medical care. When you participate, you will experience close monitoring of your condition and medications
BENEFITS
-
Advancement of medical care for yourself and others.
-
New indications for existing medications.
-
Free medical care (exams, labs, medications) pertaining to trial.
-
Reimbursement for travel and time.
RISKS
- Side effects, adverse reaction or drug interaction (monitored carefully by health care professionals).
How long does a clinical trial last?
This depends on the specific trial but anywhere from a few months to a few years.
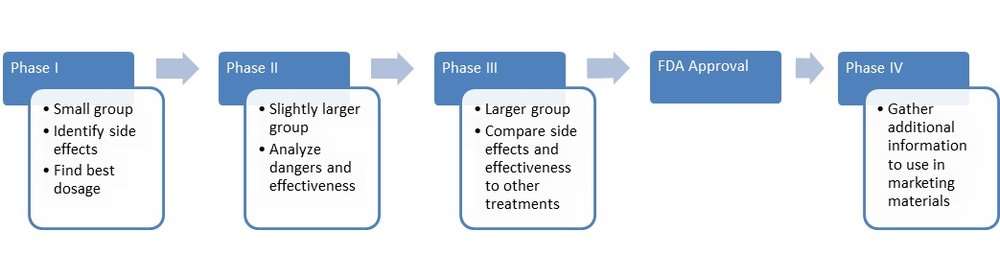
What are the risks involved?
As with any clinical trial involving a new medication or a new use of an existing medication, there are risks of adverse reactions, side effects and drug interactions. The purpose of a trial is to explore safety issues. However, most clinical trials have at least some degree of initial research previously performed on humans.
What happens if I don’t qualify for the trial?
First and foremost, we will contact your physician to advise him or her so that usual care can be provided. If you for any reason fail to qualify for a trial, you will have the option to attempt to qualify for any additional existing trial or one in the future. We just require for you to participate in one trial at a time.
Do I have to pay anything to be in the trial?
No. Study visits, exams, labs, and study related medications are provided at no cost to the patient. The patient, in most cases, will be compensated for the study visit.
Will my current doctor be made aware?
Yes. We will be in contact with your personal physician very closely to make him/her aware of your participation in the trial and any key issue during the process of the trial and study visits.
